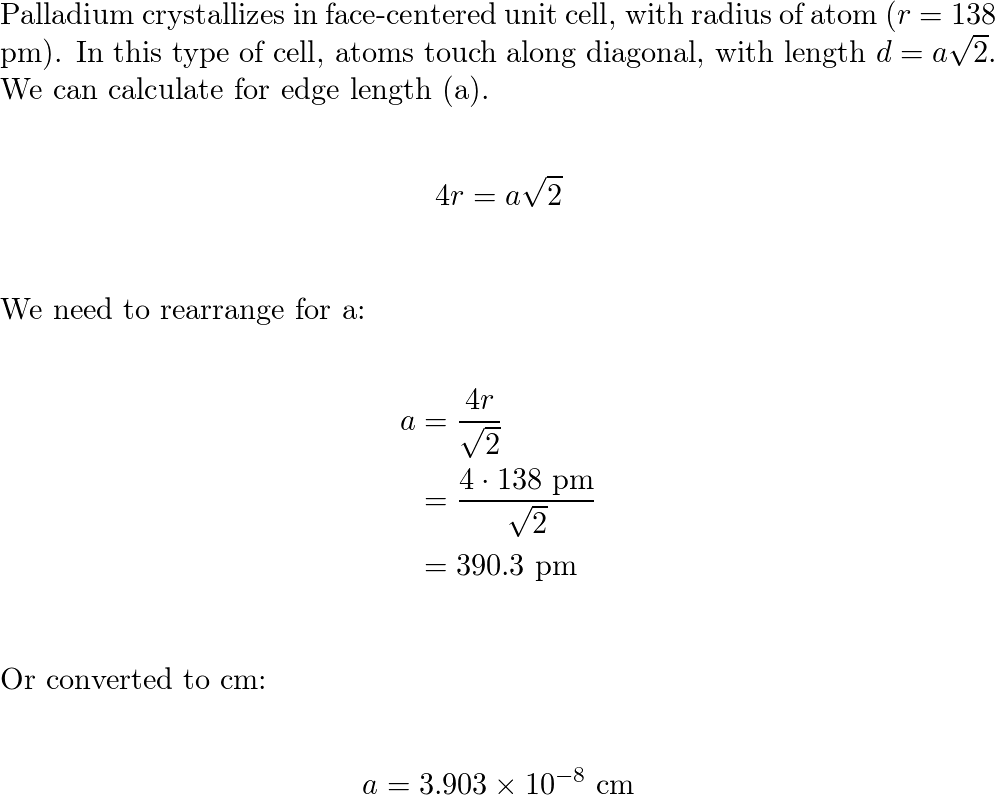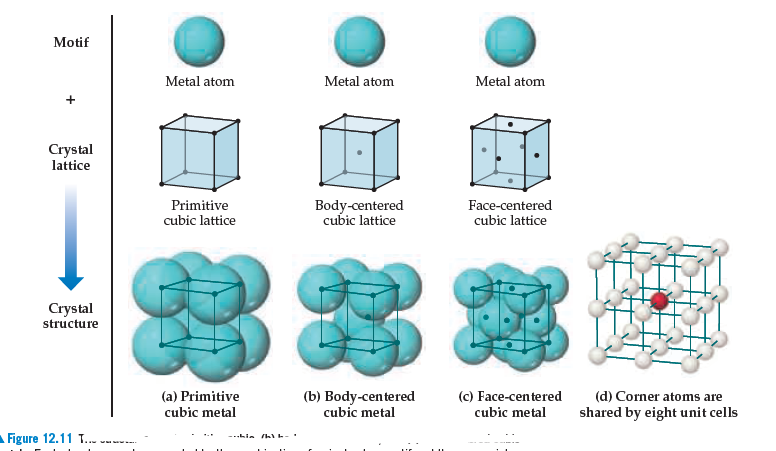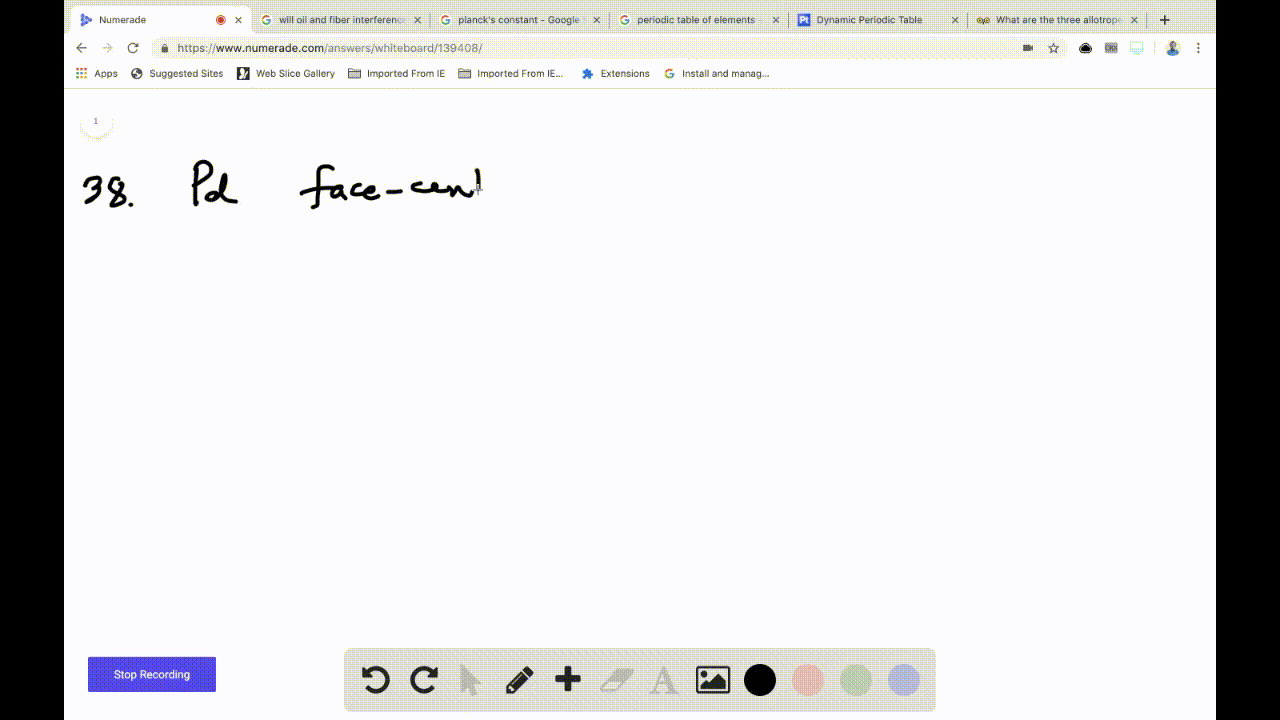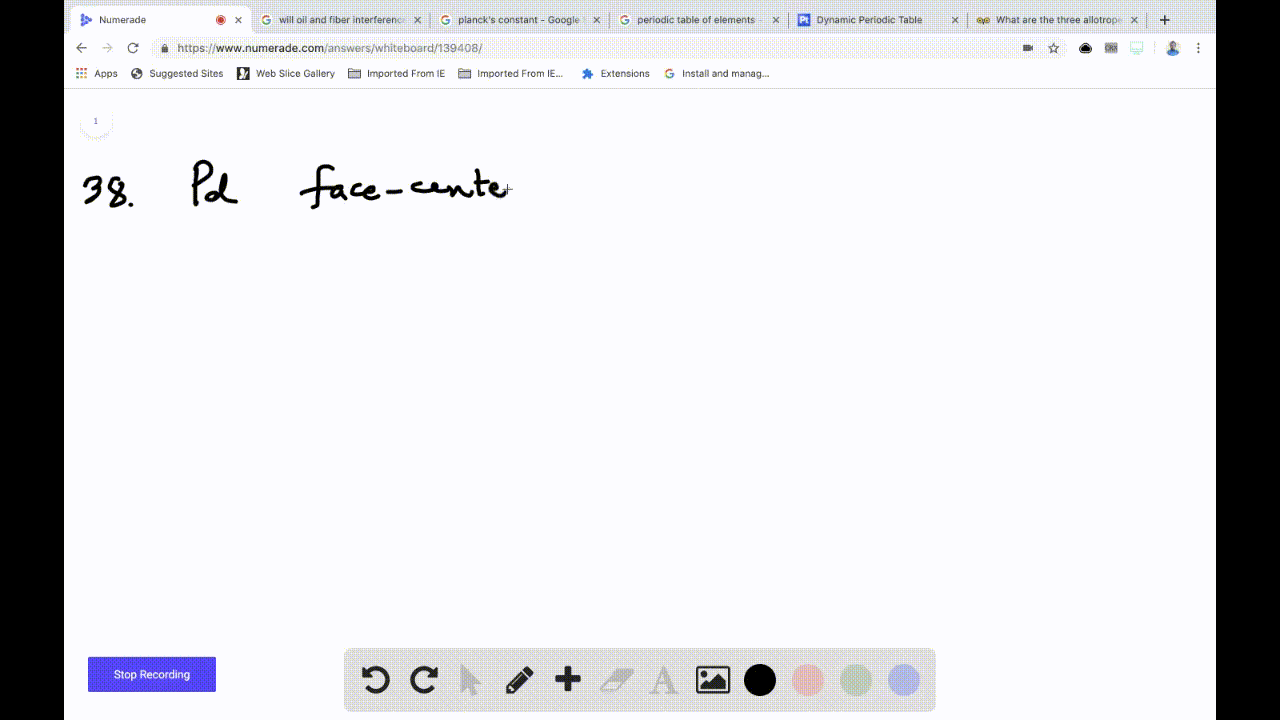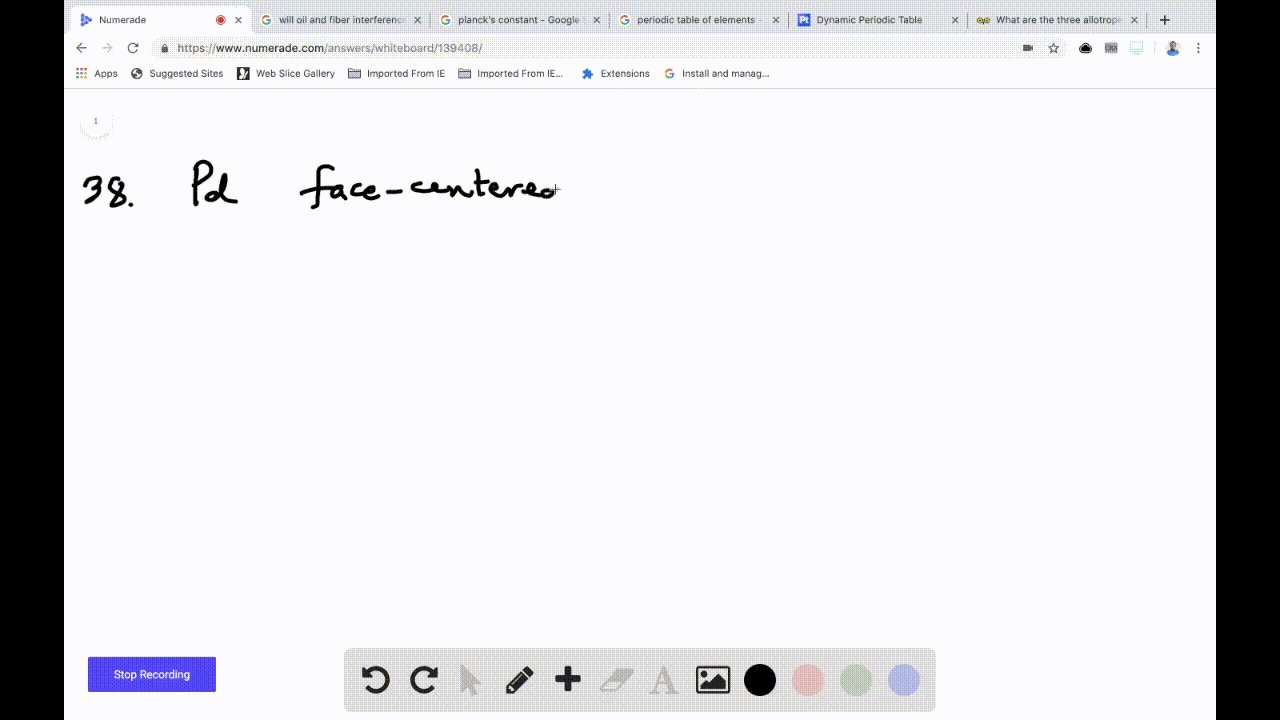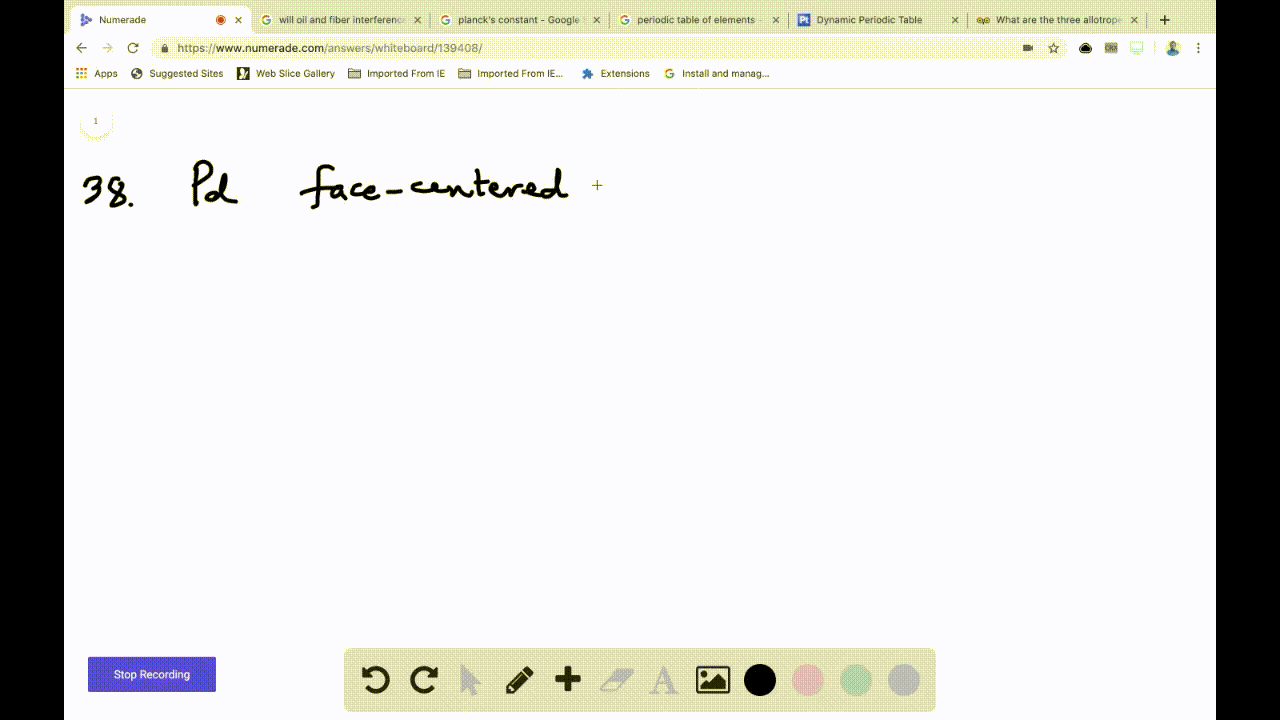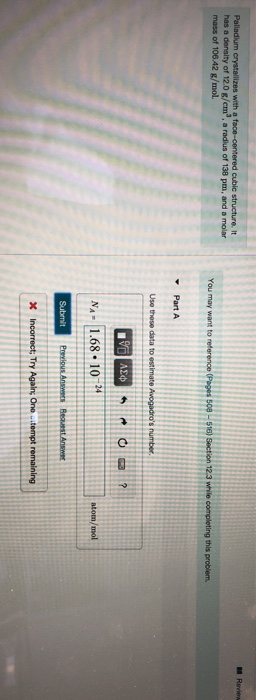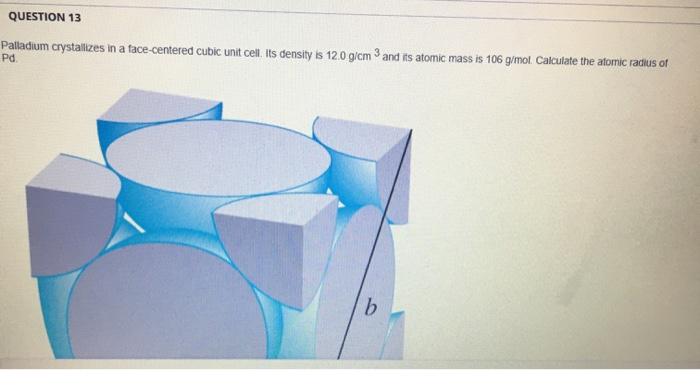
SOLVED: Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.0 g/cm³. Calculate the atomic radius of palladium: (a) 138 pm, (b) 1.95 * 10⠻⠹ nm, (c) 1.95 *

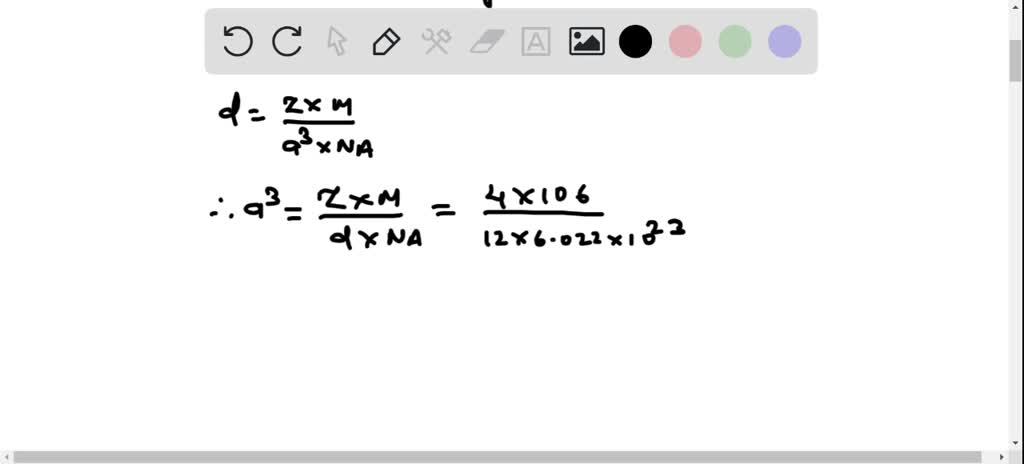
SOLVED: 1. Palladium (at. wt. = 106) crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3. Calculate the atomic radius of palladium and its packing efficiency.

A metal crystallizes in the face-centered cubic unit cell with an edge length of 320 pm. \\ A. What is the radius of the metal atom? B. The density of the metal

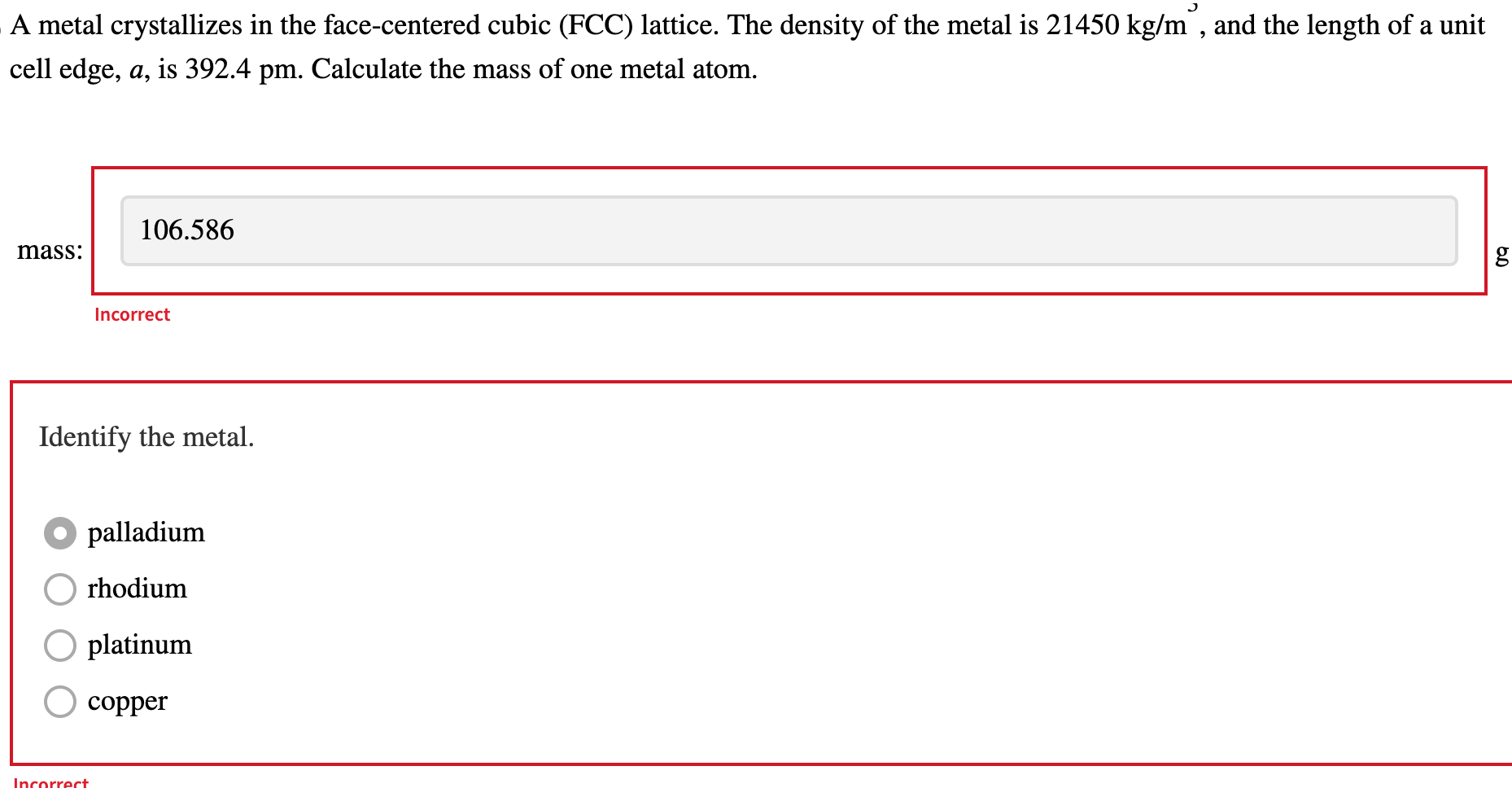
OneClass: A metal crystallizes in the face-centered cubic (FCC) lattice. The density of the metal is ...

Face-centered cubic Questions and Answers.pdf - Face-centered cubic problems Problem #1: Palladium crystallizes in a face-centered cubic unit cell. Its | Course Hero

SOLVED: 1. Palladium (at. wt. = 106) crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3. Calculate the atomic radius of palladium and its packing efficiency.